Contents
Diagnosis
The initial diagnostic approach for CAD encompasses a detailed patient history that includes compiling a comprehensive list of CAD risk factors; a thorough physical examination to include an assessment of all peripheral pulses which, when abnormal, may signal the presence of underlying peripheral arterial disease; and an electrocardiogram. Once this initial evaluation is performed, laboratory blood tests, stress testing, and a cardiac catheterization may be necessary to obtain further diagnostic insight.
History
The history should include any current symptoms and a complete inventory of comorbid conditions. An inventory of cardiac risk factors and a complete family history are essential components. The history should also include information about the character and location of discomfort; radiation of discomfort; associated symptoms; and precipitating, exacerbating, or alleviating factors. The importance of the family history should not be underestimated. A detailed assessment, particularly of first-degree relatives, for the presence of CAD and age of diagnosis is imperative when evaluating a patient’s risk-factor profile.
Physical Examination
The results of the physical examination of a patient with stable or unstable angina may be entirely normal. The presence of multiple risk factors or atherosclerosis in the carotid or peripheral arteries increases the likelihood that a chest-pain syndrome is related to myocardial ischemia. Evaluation should include measurements of blood pressure and the ankle–brachial index. Examination of the carotid arteries should include auscultation for bruits. Examination of the chest wall, neck, and shoulders for deformities and tenderness may be helpful in diagnosing musculoskeletal chest discomfort. Cardiac auscultation may detect murmurs caused by aortic stenosis or hypertrophic cardiomyopathy, either of which can cause angina in the absence of epicardial CAD. Assessment of the abdominal aorta for an aneurysm or bruits and palpation of lower extremity pulses is necessary to evaluate for peripheral vascular disease. Careful palpation of all peripheral pulses and assessment of symmetry versus diminution are also valuable noninvasive approaches for assessing the integrity of the arterial circulation. Finally, examination for xanthelasmas, tendon xanthomas, retinal arterial abnormalities, and peripheral neuropathy can be helpful.
Diagnostic and Imaging Studies
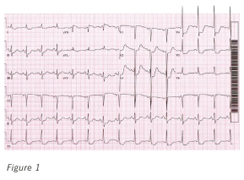
Figure 1. A 12-lead electrocardiograph of ischemic anterolateral ST-segment depression in a patient with known coronary artery disease.
Electrocardiography
A resting 12-lead electrocardiogram should be obtained on all patients with suspected CAD. Electrocardiographic results are normal in approximately 50% of patients with chronic stable angina, and they can remain normal during an episode of chest discomfort. Importantly, a normal electrocardiogram does not exclude coronary artery disease (Figure 1). When abnormal, especially when Q waves are present, a regional myocardial territory of diagnostic duration can signify the presence of a past MI with high accuracy.
Chest Radiography
The usefulness of a routine chest radiograph in a patient with chest discomfort has not been established. Calcification of the aortic knob is a common finding in older patients and is a nonspecific indicator of flow-limiting obstructive coronary disease. Coronary calcification also may be present. A widened mediastinum may signify an aortic aneurysm and represent the first clue of unstable aortic disease as the cause of chest discomfort.
Cardiac Computed Tomography Angiography
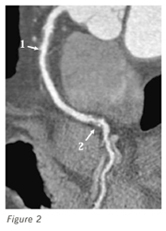
Figure 2. Computed tomography angiogram of the right coronary artery. (1. Mild proximal stenosis with expansive remodeling and predominantly nonexpansive plaque; 2. Partially calcified advanced mid to distal stenosis.)
A noninvasive imaging assessment of coronary atherosclerosis is now possible in the form of cardiac computed tomography angiography. When negative, this test possesses a high negative predictive value. The positive predictive value also is high, but exact stenosis quantification can be complicated. Associated calcification can cause a blooming artifact, resulting in an overestimation of stenosis severity (Figure 2). Additionally, previous coronary artery intervention in the form of coronary artery stent placement can create a blooming shadowing artifact rendering stenosis severity assessment within the stent challenging.
Echocardiography
Echocardiography is recommended for patients with stable angina and physical findings suggesting concomitant valvular heart disease. It is invaluable for assessing the patient with suspected hypertrophic cardiomyopathy. It also is recommended for the assessment of global and regional left ventricular systolic function in patients who have been diagnosed with congestive heart failure, complex ventricular arrhythmias, or a history of MI. The echocardiogram is in many ways an ideal test when assessing a patient with known CAD. It is painless, carries no known risk, and the results are available within approximately 30 minutes. An experienced echocardiographer can identify 1 or more MIs; localize the infarct to a coronary artery distribution; and assess for associated ischemic structural complications such as a left ventricular aneurysm, left ventricular pseudoaneurysm, and ventricular thrombus.
Laboratory Studies
Routine laboratory measurements recommended as a part of the initial evaluation of patients with CAD should include determination of fasting glucose and fasting lipid levels (total cholesterol, high-density lipoprotein [HDL] cholesterol, triglycerides, and calculated low-density lipoprotein [LDL] levels). Other markers, such as lipoprotein(a) (Lp[a]) and high-sensitivity C-reactive protein, may be useful in assessing cardiac risk. High-sensitivity C-reactive protein is gaining greater prominence in assessing the inflammatory level of vascular disease and predicting future risk of vascular events, such as MIs and cerebrovascular accidents.
This was most recently highlighted in the Jupiter Trial in which patients with a LDL cholesterol level <130 mg/dL and a high-sensitivity C-reactive protein >2.0 mg/L were randomized to rosuvastatin 20 mg/d or placebo. Those with a high-sensitivity C-reactive protein >2.0 were shown to derive benefit from rosuvastatin based on a statistically significant reduction in myocardial event rates, cardiovascular mortality, and rates of death from any cause compared to those patients who were administered placebo.
Probability of Coronary Artery Disease
Once all these initial evaluations are complete, it is possible to estimate a patient’s probability of existing CAD before proceeding with stress testing or coronary angiography (Table 2).
Table 2: Pretest Probability of Coronary Artery Disease (CAD) by Age, Gender, and Symptom Status*
| Age, yr† | Gender | Typical or Definite Angina Pectoris | Atypical or Probable Angina Pectoris | Nonanginal Chest Pain | No Symptoms |
|---|---|---|---|---|---|
| 30-39 | Male | Intermediate | Intermediate | Low | Very low |
| Female | Intermediate | Very Low | Very Low | Very Low | |
| 40-49 | Male | High | Intermediate | Intermediate | Low |
| Female | Intermediate | Low | Very Low | Very Low | |
| 50-59 | Male | High | Intermediate | Intermediate | Low |
| Female | Intermediate | Intermediate | Low | Very Low | |
| 60-69 | Male | High | Intermediate | Intermediate | Low |
| Female | High | Intermediate | Intermediate | Low | |
|
Adapted from Gibbons RJ, Balady GJ, Beasley JW, et al: ACC/AHA guidelines for exercise testing: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation 1997;96:345-354. *High probability, >90%; intermediate, 10%-90%; low, <10%; very low, <5%. †No data exist for patients younger than 30 yr or older than 69 yr, but it can be assumed that the prevalence of CAD increases with age. In a few cases, patients at the extremes of each decade may have probabilities slightly outside the high or low range. |
|||||
Stress Testing
Stress testing is another method for determining the presence of flow-limiting, functionally significant coronary artery disease. All stress-testing techniques include electrocardiography and blood-pressure monitoring. The absolute and relative contraindications to exercise stress testing are outlined in Figure 3.
Figure 3. Absolute and Relative Contraindications to Exercise Stress Testing
Absolute Contraindications |
|
Relative Contraindications |
|
|
MI, myocardial infarction. Adapted from Gibbons RJ, Balady GJ, Beasley JW, et al: ACC/AHA guidelines for exercise testing: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation 1997;96:345-354. |
Cardiovascular stress testing takes 2 forms—exercise and pharmacologic administration. The preferred method of cardiovascular stress testing is exercise, using a treadmill or bicycle. Through aerobic exercise, a higher rate pressure product (peak systolic blood pressure multiplied by peak pulse rate), and therefore greater cardiovascular stress, can be obtained. This permits an assessment of a patient’s functional capacity, providing prognostic data using the sole parameter of attained metabolic equivalents or oxygen uptake. Heart-rate recovery—how fast the heart rate decreases after exercise cessation—is also a proven and prognostically important parameter.
The most common pharmacologic agents used for nonexercise stress testing are dobutamine, dipyridamole, and adenosine or one of its derivatives. Dobutamine echocardiography is useful for determining the presence of functionally significant obstructive CAD and assessing a patient following MI, especially for the presence or absence of myocardial viability. Using echocardiography, whether it is combined with exercise or dobutamine, the physician interpreter is focusing on the global and regional endocardial thickening responses to cardiovascular stress. This technique requires significant interpreter experience as the endocardial response to dobutamine can be both subtle and transient, requiring an experienced image acquisition and image interpretation sonographer physician team.
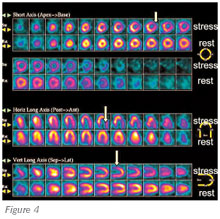
Figure 4. Myocardial perfusion scan. Stress images (arrows) demonstrate inferolateral (left circumflex) ischemia.
Nuclear stress testing is an equally important modality for assessing the coronary circulation. Unlike stress echocardiography, in which the endocardial thickening response to cardiovascular stress is the marker for inducible myocardial ischemia, nuclear stress testing relies on the concept of coronary flow reserve and differential myocardial blood flow. In the presence of exercise or the administration of a pharmacologic coronary vasodilator, the normal response is hyperemia, with a significant increase in myocardial blood flow. If there is no flow-limiting coronary obstructive disease, the pattern of hyperemia and blood flow is reflected as a symmetrical increase, with a homogeneous distribution of the blood flow tracer. In the presence of a severe flow-limiting coronary artery stenosis, dipyridamole or adenosine can induce coronary macrovascular and microvascular vasodilation, which results in differential myocardial blood flow that can be detected by radionuclide imaging with thallium 201- or technetium 99m (Tc 99m)-labeled radiopharmaceuticals (Tc 99m sestamibi or Tc 99m tetrofosmin). Functionally significant CAD can be suspected on nuclear perfusion imaging when an area of relative hypoperfusion is detected on peak stress images compared with resting images. Resting nuclear cardiac images may also be abnormal (Figure 4), signifying profound myocardial ischemia at rest or an irreversible myocardial scar consistent with past MI.
Combining imaging with the electrocardiographic stress test adds approximately 15 percentage points to the sensitivity and specificity of the test. In certain cases, electrocardiographic stress testing is of borderline help, particularly in the presence of an abnormal resting electrocardiogram. The indications for cardiac stress imaging are outlined in Figure 5.
Figure 5. Indications for Cardiac Stress Imaging
|
|
Adapted from Gibbons RJ, Balady GJ, Beasley JW, et al: ACC/AHA guidelines for exercise testing: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation 1997;96:345-354. |
Cardiac stress imaging is useful for determining the extent, severity, and location of ischemia. The exercise portion of the test also provides prognostic information. Prognostic markers include the Duke treadmill score, heart rate recovery (HRR) score, and the chronotropic response index (CRI). The Duke treadmill scoring system is summarized in Table 3.
Table 3: Duke Treadmill Scoring System*
| Risk Group | Annual Mortality Rate |
|---|---|
| Low (>4) | 0.25% |
| Intermediate (−10 to 4) | 1.25% |
| High (<−10) | 5.0% |
*The Duke treadmill score is calculated according to the following formula: Exercise time min −5 (max ST-segment deviation [in mm, during or after exercise]) −angina score where the score is 0 if there is no angina, 4 if angina occurs, and 8 if angina is the reason for stopping the test. Adapted from Mark DB, Shaw L, Harrell FE Jr, et al: Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991;325:849-853. |
|
The HRR score is calculated according to the following formula:
HRR score = HR (at peak exercise) – HR (1 min. postexercise)
where HR is in beats per minute. A normal HRR score (>12 beats/min) is associated with a low risk of death, whereas a low HRR score (<8 beats/min) is associated with a high risk. HRR scores of 8 to 12 beats per minute indicate an intermediate risk.
The CRI is calculated according to the following formula:
(Peak HR – resting HR)/([220 – patient’s age] – resting HR)
where HR is in beats per minute. A normal CRI (>0.8) is associated with a decreased probability of coronary artery disease and a lower risk of death. A low CRI (<0.8) in a patient who is not on beta-blocker therapy is associated with an increased likelihood of coronary artery disease and a higher risk of death.
Additional testing includes positron emission tomography (PET) imaging and cardiac magnetic resonance imaging (MRI). PET imaging is a form of pharmacologic nuclear cardiac stress testing that uses a coronary artery myocardial perfusion agent in the form of rubidium and 18F-deoxyglucose, which can assess myocardial metabolic activity. This test can be extremely helpful in assessing patients with ischemic heart disease, a past MI, and the extent of myocardial scar versus myocardial viability. Similarly, MRI with gadolinium also can be a useful modality to assess both the extent and location of ischemic myocardial dysfunction and myocardial viability, with MRI gaining in clinical use where offered, most typically in specialty centers.
Coronary Arteriography
Cardiac catheterization remains the gold standard for determining the presence of obstructive CAD. A cardiac catheterization yields a 2-dimensional rendering of the coronary artery circulation. To assist in circumventing the limitations of a 2-dimensional depiction of 3-dimensional anatomy, multiple views from varying angles are obtained with the extent of CAD severity typically ascribed to the angulation with the greatest stenosis severity within the particular coronary arterial segment.
